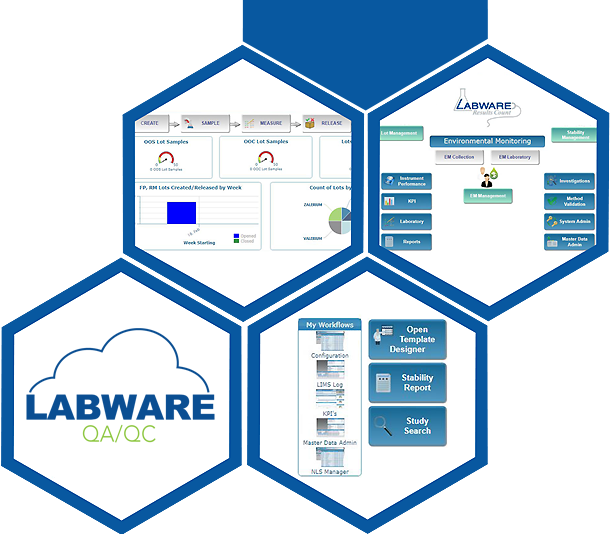
LabWare QA/QC
LabWare`s QA/QC SaaS product can get your labs running the market leading LIMS system in the shortest timeframe. Fully compliant workflows and reports are built in to give you access to the rich feature set of LabWare`s Solution.QA/QC is designed to support usage once your Master Data is loaded. User Guides and Training will have your labs running at your pace. Instrument Integration will reduce, eliminate transposition errors and delays.
Get Started with your subscription today!
LabWare QA/QC
Features
KPI Dashboards
LabWare QA/QC has specially designed KPI dashboards that help you manage your lab operations. Know what`s happening in your labs and react based on real time data. Review productivity and identify bottlenecks in order to optimize lab efficiency.
Regulatory Compliance
LabWare QA/QC has been designed from the ground up with the pharmaceutical industry in mind. In order to achieve this, LabWare LIMS has been equipped with a wide of features to facilitate compliance with regulatory guidelines and rulings, such as GMP, GLP, GCP and others (generally grouped together as GxP). In addition, other regulations such as 21 CFR Part 11, ISO 17025 and various national rulings are comprehensively addressed with LabWare LIMS.
Lot Management and Release
The capabilities of LabWare`s QA/QC enable you to manage the materials, testing and release of data for lot-based workflows and manufacturing processes. LabWare`s solution helps streamline testing of raw materials, in process and finished product samples, while maintaining all of the essential data associations needed to support where-used analysis, lot genealogy and annual review reporting. Review and release actions for Lots or Batches can be performed according to multiple market specifications, optimizing the lot disposition process.
Optimized User Interface
Visual Workflows guide users through the processes related to their role, reducing errors and increaseing efficiency. Intuitive views of information and system functions promote user adoption of the system, keeping your key people resources engaged and productive. Reduce your training times and costs and increase user accuracy with the ready to use interfaces provided with LabWare`s QA/QC.
Environmental Monitoring
LabWare`s QA/QC includes integrated EM protocols for both routine and ad hoc scheduling operations as well as gowning qualification. Pre-logged samples based on flexible specifications allow for load balancing and save valuable time through automation of routine processes.
Flexible Reporting
A complete report library is built in to QA/QC. The LabWare solution offers a unique secure reports function managed reporting requirements. Everything from Certificates Of Analysis to Summary Reports, Progress Reports and Audit Reports, all are handled accurately and efficiently.